LGMD2I is autosomal recessive muscular dystrophy caused by pathogenic mutations in the FKRP gene. The clinical presentation is very variable with individual losing ambulation as early as their early teens to as late as their 70s. The medical specialty providing care to people affected with muscular dystrophy is Neurology.
In LGMD2I, the number 2 means that the inheritance is recessive (see “Inheritance”), and the letter “I” has no meaning other than to help differentiate this condition from others due to mutations in different genes. This nomenclature has recently been reassessed in a ENMC workshop, and the recommendation of the workshop should be out soon.
From Mutation to Muscle Weakness:
FKRP means Fukutin Related Protein. In 2016, it was discovered that FKRP modifies a protein that resides in the membrane of our muscles. It has been suggested that the modifications by FKRP of the membrane protein are critical to maintain the integrity of the muscle during the contraction-relaxation cycle, which muscles undergo when a person moves, stands, or breaths.
In people with LGMD2I, the FKRP gene is mutated in a manner that impacts the functionality of the protein it codes for. The FKRP protein is created with a kink, which dramatically lowers its efficiency. Ultimately, FKRP cannot modify its target protein, which is thought to be the main reason why muscles in LGMD2I are brittle and degenerate at a larger rate than they do in people without mutations in the FKRP gene.
Brittle Muscles:
As explained in the section The Muscle, all myofibers are enveloped by the basement membrane. For the basement membrane to strengthen the fibers and save them from injury during movement, it has to be chemically bound to the fiber. One of the main proteins of the myofibers that crosslinks with the basement membrane is called alpha-dystroglycan (aDG), and it needs to be modified by FKRP to be functional. It is suggested that abnormal FKRP function fails to modify aDG, which cannot crosslink with the basement membrane, increasing the myofiber vulnerability to breakage during cycles of contraction/relaxation.
Loss of membrane integrity due to contraction-induced damage eventually leads to muscle degeneration over time.
Illustration to compare the consequences of injury in healthy muscles and dystrophic muscles (from doi: 10.1186/2044-5040-1-21)
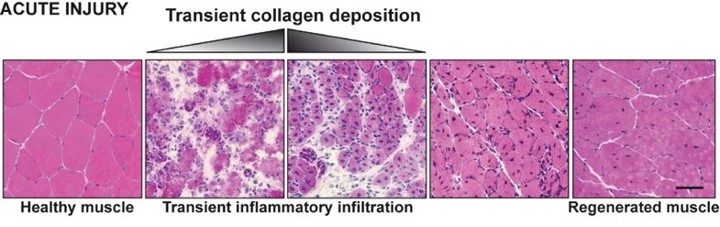
Acute injury to healthy muscle produces transient collagen deposition (in white) an rapid and controlled inflammation that removes dead and damaged myofibers, and promotes replacement of the injured muscle.
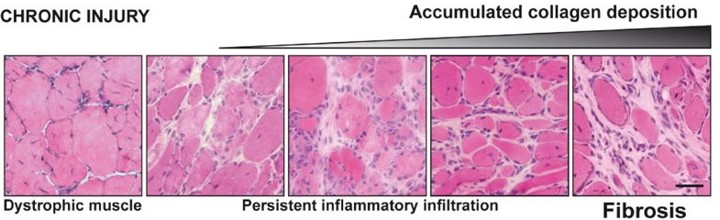
In conditions of chronic injury, as occurs in the muscular dystrophies, chronic inflammatory events result in the excessive accumulation of ECM components, which inhibit myogenic repair and lead to muscle being replaced by fibrotic/scar tissue.
Disease Manifestation:
It is not understood why some muscle groups are more affected by the lack of FKRP function than others. And in the same muscle group, some muscles will undergo degeneration much quicker and more extensively than other muscles. The muscle weakness in LGMD2I first affects the ability to raise from the floor and to climb stairs, but it also has an early impact on the ability to maintain the body’s balance. Physical therapy, exercise and some alternative therapies are thought to help people affected by LGMD2I slow down the loss of these abilities.
Cardiac and respiratory complications:
In addition to the muscles of the girdle, the diaphragm and the heart muscle are affected. The extent and the pace with which these 2 organs are affected are not predictable. However, because it is a common feature of LGMD2I, everybody diagnosed with pathogenic mutations in the FKRP gene should visit with a cardiologist and a pulmonologist regularly.
Because cardiac and respiratory insufficiencies can occur before loss of ambulation, it is prudent to perform cardiac and respiratory evaluation at the time of diagnosis. One important outcome of the first meeting with these specialists should be a baseline measure of your respiratory and cardiac capacities.
People may not realize the significant loss of cardiac and respiratory functions until they are quite advanced.
- Respiratory insufficiency causes nocturnal hypoventilation, a condition that creates, among other symptoms, a general sense of lethargy and drowsiness during the day as well as fatigue and morning headaches.
- Some of the signs of dilated cardiomyopathy are shortness of breath, fatigue, palpitations, light-headedness, and chest pain.